Published On Mar 10, 2021
This lecture is about Pauli exclusion principle and spin of electrons in orbitals.
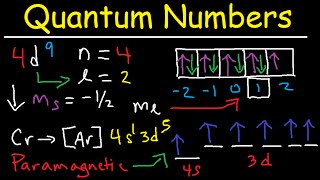
Q: What is Pauli exclusion principle?
Ans: Pauli exclusion principle is defined as no two electrons in an atom have the same set of four quantum numbers. Also, you can define Pauli exclusion principle as each orbital can accommodate only two electrons with opposite spin.
To learn more about Pauli exclusion principle, watch this animated lecture till the end.
#Pauli_Exclusion_Principle
#chemistry
Subscribe my channel at: / @najamacademy
Youtube link: / @najamacademy
Facebook link: / najamacademy
show more