Published On Mar 1, 2021
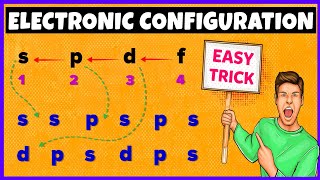
This lecture is about aufbau principle in chemistry and how to find the energy of orbitals. Also, you will learn the easy trick of remembering aufbau principle by knowing the energy of orbitals.
Q: What is aufbau principle in chemistry?
Ans: Aufbau principle states that electrons fill the lowest energy levels before occupying the highest energy orbitals.
We know that the atomic number of sodium is 11. We can easily configure these 11 electrons in orbitals using aufbau principle.
For example, firstly electron of the sodium will fill the 1s orbital, then 2s orbital, then 2p orbital and then 3s orbital.
To learn more about aufbau principle, watch this animated lecture till the end.
#aufbauprinciple
#aufbauprinciplediagram
#chemistry
Subscribe my channel at: / @najamacademy
Youtube link: / @najamacademy
Facebook link: / najamacademy