Published On Premiered Dec 22, 2021
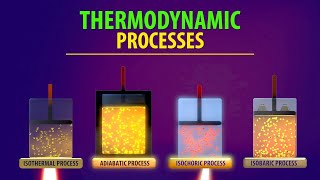
Thermodynamics is the study of the relations between heat, work, temperature, and energy. The laws of thermodynamics describe how the energy in a system changes and whether the system can perform useful work on its surroundings.
Section Summary. The first law of thermodynamics is given as ΔU = Q − W, where ΔU is the change in internal energy of a system, Q is the net heat transfer (the sum of all heat transfer into and out of the system), and W is the net work done (the sum of all work done on or by the system).
An extensive property is a property that depends on the amount of matter in a sample. ... An intensive property is a property of matter that depends only on the type of matter in a sample and not on the amount. Color, temperature, and solubility are examples of intensive properties.
An extensive property of a system depends on the system size or the amount of matter in the system. If the value of the property of a system is equal to the sum of the values for the parts of the system then such a property is called extensive property. Volume, energy, and mass are examples of extensive properties.
Intensive properties do not depend on the amount of the substance present. Some examples of intensive properties are color, taste, and melting point. Extensive properties vary according to the amount of matter present. Examples of extensive properties include mass, volume, and length.
Thermodynamic properties are defined as characteristic features of a system, capable of specifying the system's state. ... "Specific" properties are expressed on a per mass basis. If the units were changed from per mass to, for example, per mole, the property would remain as it was (i.e., intensive or extensive).