Published On Oct 18, 2022
Learning Competency Use mole fraction and molality in expressing the concentration of solutions (STEM_GC11PP- IIId-f-111)
Specific Learning Outcomes At the end of the lesson, the learners will be able to:
• express the concentration of solutions in mole fraction and molality; and
• perform calculations for solution concentration given appropriate data.
Quantitative study of a solution requires knowing its concentration, that is, the amount of solute present in a given amount of solution. Chemists use several different concentration units, each of which has advantages as well as limitations.
What is percent by mass?
The percent by mass (also called percent by weight or weight percent) is the ratio of the mass of a solute to the mass of the solution, multiplied by 100 percent.
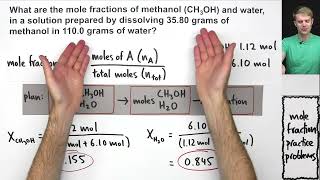
What is a mole fraction?
Mole fraction is a unit of concentration, defined to be equal to the number of moles of a component divided by the total number of moles of a solution.
What is molality?
molarity was defined as the number of moles of solute in 1 L of solution
What is molality?
Number of moles of solute dissolved in 1 kg (1000 g) of solvent
Concentration Units Exercises
- The density of a 2.45 M aqueous solution of methanol (CH3OH) is 0.976 g/mL. What is the molality of the solution? The molar mass of methanol is 32.04 g.
- Calculate the molality of a 35.4 percent (by mass) aqueous solution of phosphoric acid (H3PO4). The molar mass of phosphoric acid is 97.99 g.